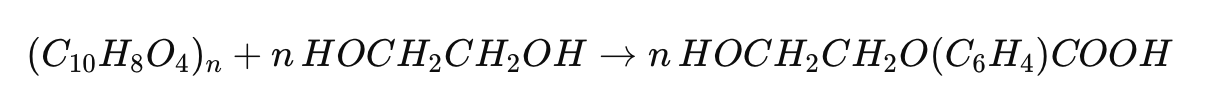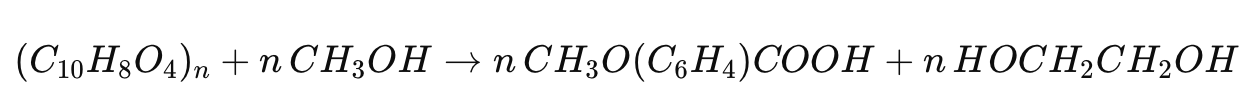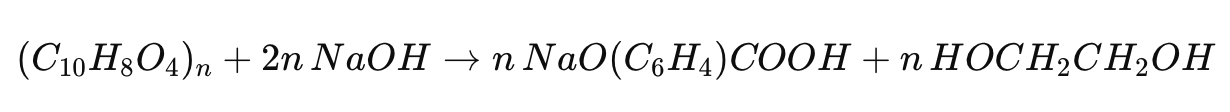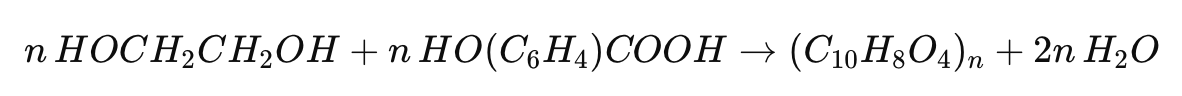How are PET Recycled? From Waste to Resource
Polyethylene terephthalate, commonly known as PET, is a type of plastic widely used in the manufacturing of beverage containers, including cups. While PET cups offer convenience and durability, their disposal poses significant environmental challenges. However, the recycling process of PET cups provides a promising solution to reduce waste and conserve resources. In this blog, we will delve into the intricate process of PET cup recycling, examining chemical formulas, data, and research findings to understand how these everyday items are transformed from waste to valuable resources.
1. Understanding PET and Its Properties
PET, with the chemical formula (C10H8O4)n , is a type of polyester derived from terephthalic acid and ethylene glycol. It is valued for its strength, thermo-stability, and transparency, making it an ideal material for beverage containers. The widespread use of PET cups, however, leads to a substantial amount of plastic waste, necessitating efficient recycling methods.
2. Collection and Sorting
The recycling process begins with the collection and sorting of PET cups. Efficient collection systems, such as curbside recycling programs and deposit-return schemes, play a crucial role in gathering used PET cups. Once collected, the PET waste undergoes a sorting process to separate it from other types of plastics and contaminants. Advanced technologies, such as Near-Infrared (NIR) spectroscopy, are employed to accurately identify and sort PET materials.
3. Washing and Cleaning
After sorting, the PET cups are washed to remove labels, adhesives, and residual contents. This step is critical to ensure the purity of the recycled material. The washing process typically involves the use of alkaline or acidic solutions, which help in breaking down and removing contaminants. Research indicates that an efficient washing process can achieve a reduction of up to 95% in contaminant levels, thereby enhancing the quality of the recycled PET (rPET).
4. Shredding and Size Reduction
Once cleaned, the PET cups are shredded into small flakes. This size reduction is essential for the subsequent stages of recycling. The shredding process increases the surface area of the PET material, facilitating easier handling and processing. According to studies, the optimal size of PET flakes for recycling ranges between 6-12 mm, as this size allows for efficient melting and reformation.
5. Depolymerization
Depolymerization is a key chemical process in PET recycling. It involves breaking down the long polymer chains of PET into its monomers, terephthalic acid (TPA) and ethylene glycol (EG). This can be achieved through various methods, including glycolysis, methanolysis, and hydrolysis.
Glycolysis
In glycolysis, PET reacts with an excess of ethylene glycol in the presence of a catalyst, typically zinc acetate, at temperatures ranging from 180°C to 240°C. The chemical equation for glycolysis is:
This process breaks down PET into bis(2-hydroxyethyl) terephthalate (BHET), which can be further purified and used to synthesize new PET.
Methanolysis
Methanolysis involves the reaction of PET with methanol at elevated temperatures and pressures, using catalysts such as zinc or cobalt acetate. The chemical equation for methanolysis is:
This process produces dimethyl terephthalate (DMT) and ethylene glycol, both of which can be repolymerized to form new PET.
Hydrolysis
Hydrolysis, either acidic or alkaline, breaks down PET into terephthalic acid and ethylene glycol. Alkaline hydrolysis uses sodium hydroxide (NaOH) as a catalyst:
This process yields sodium terephthalate and ethylene glycol, which can be converted back into PET.
6. Polymerization
The purified monomers obtained from depolymerization undergo polymerization to form new PET. The process involves the reaction of terephthalic acid or its derivatives with ethylene glycol, resulting in the formation of PET through a condensation reaction:
This step restores the PET to its original properties, enabling the production of new PET cups and other products.
7. Melt Processing
The newly formed PET undergoes melt processing to create pellets or granules, which serve as raw material for manufacturing new products. Melt processing involves heating the PET to its melting point, followed by extrusion and cooling to form uniform pellets. These pellets are then used in the production of new PET cups, bottles, and other items.
8. Quality Control and Testing
Quality control is essential to ensure that the recycled PET meets industry standards and regulations. Tests such as intrinsic viscosity (IV) measurement, differential scanning calorimetry (DSC), and mechanical property evaluation are conducted to assess the quality and performance of rPET. Studies have shown that high-quality rPET can exhibit properties comparable to virgin PET, making it suitable for a wide range of applications.
9. Environmental and Economic Benefits
Recycling PET cups offers significant environmental and economic benefits. According to research, recycling PET can reduce greenhouse gas emissions by up to 60% compared to producing virgin PET. Additionally, it conserves resources by reducing the demand for petroleum-based raw materials. Economically, the recycling industry creates jobs and generates revenue, contributing to a sustainable economy.
The recycling of PET cups is a complex but highly beneficial process that transforms waste into valuable resources. Through a series of steps, including collection, sorting, washing, depolymerization, and polymerization, PET cups are converted back into raw materials for new products. Chemical processes like glycolysis, methanolysis, and hydrolysis play a crucial role in breaking down PET into its monomers, enabling the production of high-quality recycled PET. By understanding and supporting the recycling process, we can significantly reduce plastic waste, conserve resources, and promote a more sustainable future. To seek high quality recycled PET resin that is both durable and sustainable, try Wankai PET.